
LIFE SCIENCES
Built for today’s scientific velocity and regulatory pressure
Don’t let workforce challenges slow your innovations
To compete in a high-stakes, high-oversight industry, life science companies must rapidly skill and certify employees, ensure compliance, and stay ready for every inspection, innovation, and shifts in standards.
Outpace regulatory change
Constant shifts in regulatory expectations make it difficult to keep training consistent and audit-ready. Without scalable workflows, organizations face higher risk of delays and findings.
Adapt to talent shortages
Life science roles are hard to fill, and new technologies require new skills. Limited skill development slows onboarding and impacts productivity.
Align and localize learning
Global R&D, pharma, clinical and medical device manufacturing teams often train in disconnected systems. Without a single source of truth, it's harder to deliver accurate, localized training that supports fast market entry.
Innovate with Cornerstone
Stay audit-ready with smarter compliance
Deliver consistent learning across roles and geographies by producing real-time audit reports that keep you inspection ready.



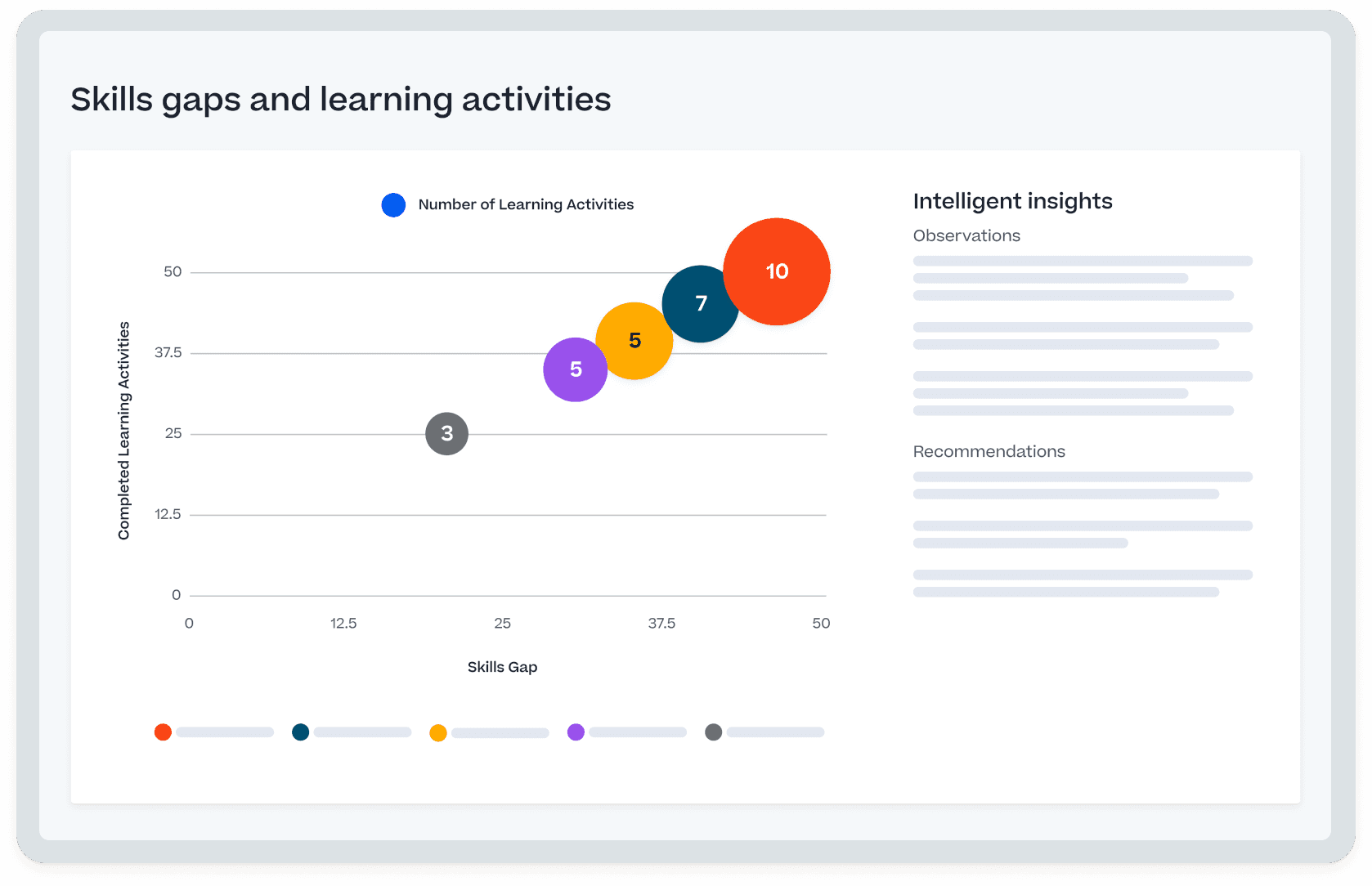
Our commitment to life science customers
Dedicated expertise, validation support, and partnership help you meet evolving regulatory and operational expectations.
System validation services
We produce validation deliverables with every release that are aligned with GAMP 5, IEEE 1012 and the FDA Computer Software Assurance (CSA) Guidance allowing you to leverage and focus on your intended use.
Regulatory industry expertise
Our Quality and Risk Management team brings deep life science, healthcare, and manufacturing expertise to ensure applicable GxP controls are integrated into our platform and processes.
Life science forum
We have a dedicated life science forum that consists of a steering committee with customers and Cornerstone quality and product leadership. This allows the industry to speak in one voice and to influence our product roadmap.
Proven impact across readiness, performance, and ROI
Independent analysis shows measurable gains in efficiency, workforce readiness, and overall business value
$7.4M
Compliance savings over three years by improving life sciences training, tracking, and safety
Forrester Total Economic Impact™ of Cornerstone (2024)
40%
Faster time to productivity with more efficient, role-based learning
Forrester Total Economic Impact™ of Cornerstone (2024)
443%
Average three-year ROI reported by Cornerstone customers
Forrester Total Economic Impact™ of Cornerstone (2024)

Real-life success stories from Cornerstone customers
Results
An improved and simplified learning and development experience with an LXP that streamlined and consolidated mandated and skill-based content – resulting in improved compliance and validation, increased engagement, and collectively increased competence.

Extend readiness across your life science ecosystem
Be sure your distributors, HCPs, CROs, CMOs, suppliers and global partners stay aligned with and trained on your quality, safety, and regulatory standards.
Maintain global training consistency
Deliver validated, auditable life science training to external partners with the same rigor as internal teams. Ensure every CRO, CMO, distributor, or field team receives accurate SOP, GxP, and safety content everywhere in the world.
Reduce compliance risk across your ecosystem
Standardize partner training, certification, and documentation to strengthen audit defensibility and protect regulatory standing across global supply and research networks.
Accelerate speed to market
Onboard and certify external teams faster with automated workflows, role-based assignments, and unified reporting. Ensure every partner contributing to R&D, manufacturing or commercial operations is ready to execute without delays.
FAQ
Why should I choose Cornerstone for life science training and talent management?
Cornerstone is an AI-powered, validated learning and talent platform built for pharmaceutical, biotech, and medical device organizations. It supports GxP compliance, 21 CFR Part 11, and global audit expectations while helping teams develop the skills required for safe, efficient, and inspection-ready operations.
How can I demonstrate LMS ROI in life science?
Organizations see ROI through shorter onboarding times and improved productivity. In addition, by leveraging the Cornerstone validation, internal efforts can be significantly reduced.
How does the Cornerstone LMS comply with Part 11 Electronic Records and Electronic Signatures?
The Cornerstone LMS has the technical controls to meet 21 CFR Part 11 compliance through configuration (e.g., Electronic Signatures, audit trail). Most technical controls are met by the software, but procedural controls are often required by Cornerstone and the regulated customer.
How does Cornerstone improve onboarding for new life science employees?
Structured, role-based onboarding aligns to SOPs and compliance requirements. Day-one assignments are automated and completions are tracked for operational and audit purposes.
What computer system validation deliverables are available for each release?
Cornerstone clearly understands that validation at implementation and maintaining a validated state during times of change is critical for our regulated Customers. To assist with this effort, we offer IV&V (Independent Verification & Validation) Maintenance Service which provides key validation deliverables to customers for each release which can be leveraged during validation activities.
Related Resources
Want to keep learning? Explore our products, customer stories, and the latest industry insights.

Book a Demo
Find out why more than 7,000 organizations and 125 million users in 180 countries use Cornerstone Galaxy to build high-performing, future-ready people and organizations today.















